Operating system: Windows, Android, macOS
Group of programs: Business automation
Quality control of laboratory tests
- Copyright protects the unique methods of business automation that are used in our programs.

Copyright - We are a verified software publisher. This is displayed in the operating system when running our programs and demo-versions.

Verified publisher - We work with organizations around the world from small businesses to large ones. Our company is included in the international register of companies and has an electronic trust mark.

Sign of trust
Quick transition.
What do you want to do now?
If you want to get acquainted with the program, the fastest way is to first watch the full video, and then download the free demo version and work with it yourself. If necessary, request a presentation from technical support or read the instructions.

Contact us here
How to buy the program?
View a screenshot of the program
Watch a video about the program
Download demo version
Compare configurations of the program
Calculate the cost of software
Calculate the cost of the cloud if you need a cloud server
Who is the developer?
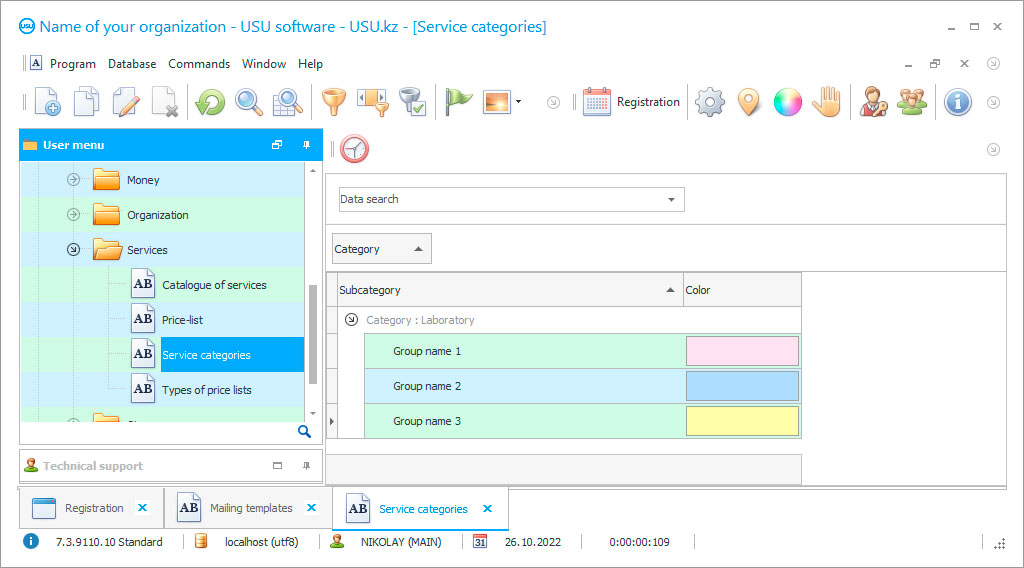
Program screenshot
Quality control of laboratory tests should begin with the standardization of the activities of the laboratory institution. Strict work regulations will allow the use of automation tools for all activities in general and for quality control as applied to laboratory tests. The technological stages of automation of quality control in clinical laboratory studies are clear — biological material is supplied to examination, the receipt is accompanied by a parallel information flow in the form of information about a specific patient, the type of test required, methods of analyzing biological material; then the control process begins, accompanied by the receipt of information about the study from chemical analyzers; based on the final laboratory test data, forms of the analysis results are prepared; financial and financial document flow is created automatically in a unified form, statistical information is stored to the formation of management reporting and the creation and control of an archive database.
Process automation is gaining momentum, but undeveloped healthcare facilities still do most of the operations manually, often re-inventing the wheel over and over again. It should be noted that harmonization should extend not only to the control function within the laboratory but also to the processes of the client institutions. A huge help in this matter is the standards in the practice of clinical trials that do not allow discrepancies in the organization of activities: recommendations of the International Organization of Standardization, and national regulatory documents, such as state standards, instructions, and orders of the Ministry of Health, etc.
Who is the developer?

Akulov Nikolay
Expert and chief programmer who participated in the design and development of this software.
2024-05-18
Video of quality control of laboratory tests
This video can be viewed with subtitles in your own language.
Software developers, having a clear description of the control steps, produce programs for control of laboratory research. Quality control is the most automated area of software developments today. Correctly and timely performed the analysis at a sufficiently high analytical level with the availability of information required for the interpretation of the test is the basis for quality control as applied to a clinical laboratory test. This process is almost impossible without a reliable control tool created through a quality assurance system in laboratory clinical trials.
Such a tool will make it possible to timely identify erroneous deviations that inevitably arise in the work of clinical diagnostic enterprises, as in any field of human activity, to carry out targeted measures in order to reduce the possibility of wrong data to a minimum. A set of systematically planned monitoring measures provides a high level of confidence in achieving the required quality level at each stage of the patient examination process when each separately taken authorized report on the analysis carried out in the laboratory can be confidently used by the doctor in the diagnosis and in the preparation of the treatment schedule.
Download demo version
When starting the program, you can select the language.
Who is the translator?

Khoilo Roman
Chief programmer who took part in the translation of this software into different languages.
The quality of the results of examinations and analyzes underlies the current and future state of the patient. The quality of clinical diagnostics is directly and directly influenced by such factors as professionalism and the availability of a sufficient number of qualified medical personnel, the level of funding of a medical institution, as well as the quality of building a system of activities: stages of analytic, examination elements, the composition of reporting, level of interpretation of analyzes, an advisory component of patient care.
Quality control of clinical laboratory tests is carried out in real-time on an ongoing basis by means of software for automating the activities of the clinical diagnostic laboratory. The program is easy to use for laboratory users and does not require any specialized training. The aesthetically pleasing and logical interface supports the work of the staff in the most friendly manner. Information databases are reliably protected by a system of logins and passwords, each of the users has an individual level of access to the databases, depending on the range of duties and areas of responsibility. The management reporting system for test control indicators applied to the quality of each clinical laboratory study is built on a statistical database that is constantly updated with up-to-date information on the activities of the laboratory. Test reports are automatically generated at the request of users of any access level, the schedule for submission and the composition of the reports can be compiled according to the needs of the enterprise. Customer convenience is thought out to the smallest detail. The client can download the test results from the laboratory's website using any electronic device by going to his personal account. Personal information is controlled by the system and reliably protected by the most modern software tools. Payment by the client can be made from any nearest payment terminal. Information about the transfer of funds by the client immediately enters the laboratory's database.
Order a quality control of laboratory tests
To buy the program, just call or write to us. Our specialists will agree with you on the appropriate software configuration, prepare a contract and an invoice for payment.
How to buy the program?

The program will be installed
If the program is purchased for 1 user, it will take no more than 1 hourBuy a ready-made program
Also you can order custom software development
If you have special software requirements, order custom development. Then you won’t have to adapt to the program, but the program will be adjusted to your business processes!
Quality control of laboratory tests
The quality of work is controlled on the basis of the most modern standards, the latest laws, instructions, and orders developed by the health authorities.
The strictest requirements are imposed on the quality of laboratory materials, reagents, and equipment. Tests are continuously monitored using the program by authorized laboratory personnel. Preventive maintenance of technical laboratory equipment is carried out in a timely manner, only materials and reagents that have been tested for compliance with generally accepted standards with current expiration dates are allowed to work.
The possibility of seamless integration of IT tools with the laboratory material and technical base of medical institutions participating in the analytical stages of work is provided. Adaptation does not require any special settings by the efforts of system administrators, and additional expenses are not required for the purchase of special equipment to work with automation tools.